
SurGenTec Gains FDA Clearance for OsteoFlo® HydroFiber™ for Stand-Alone Use
SurGenTec is excited to announce that its innovative product, OsteoFlo HydroFiber, has received FDA 510(k) clearance, marking a major milestone in the evolution of bone graft technology. This clearance establishes OsteoFlo HydroFiber as a stand-alone solution, equivalent to autograft, for spine surgeries. It is now approved for use in interbody fusion cages, disc spaces, and posterolateral fusions, offering a significant advancement in spinal fusion procedures.
OsteoFlo HydroFiber: A Game-Changer in Bone Graft Technology
SurGenTec is revolutionizing bone graft technology with the introduction of OsteoFlo HydroFiber, a groundbreaking product that has received FDA 510(k) clearance for use in spine surgeries. This new synthetic bone graft option is now recognized as a stand-alone solution equivalent to autograft, and it is suitable for a variety of procedures, including interbody fusion cages, disc spaces, and posterolateral fusions. OsteoFlo HydroFiber represents a significant advancement in orthopedic and spine surgery, providing surgeons with an innovative and effective tool to enhance patient outcomes.
Innovative Technology Behind OsteoFlo HydroFiber
At the heart of OsteoFlo HydroFiber’s effectiveness is its unique Web Interlace Technology. This technology suspends particles within its fibers, preventing graft migration while ensuring optimal cohesiveness and flowability. The product’s hydrophilic bonds and proprietary fibers are designed to absorb saline, blood, or bone marrow aspirate, creating a stable environment that supports the healing process. By offering a cohesive platform that can absorb vital fluids, OsteoFlo HydroFiber helps provide a foundation for bone growth and healing while maintaining stability throughout the surgical procedure.
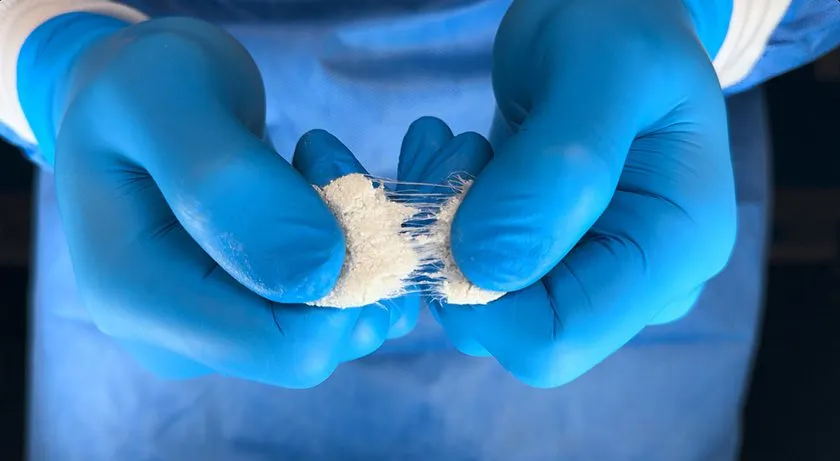
OsteoFlo HydroFiber is not only an advanced synthetic bone graft but also pairs seamlessly with SurGenTec’s flagship delivery device, the Graftgun. This combination enables efficient backfilling of interbody cages, marking a significant improvement over traditional funnel methods. Surgeons can now achieve more accurate and effective graft placement, enhancing the precision and success of spine surgeries.
Safer, Smarter Solutions for Surgeons and Patients
One of the most significant benefits of OsteoFlo HydroFiber is its ability to provide an effective alternative to human tissue. By reducing the reliance on allografts, OsteoFlo HydroFiber eliminates the risks associated with donor variability, disease transmission, and storage complications. The need for autograft harvesting is also eliminated, which reduces surgical time and minimizes the potential for painful recovery. This innovation allows patients to experience improved outcomes with fewer complications and faster recovery times.
“This product is set to revolutionize bone graft technology,” said Travis Greenhalgh, CEO and founder of SurGenTec. “OsteoFlo HydroFiber provides a novel, customizable solution for surgeons, tailored to both anatomy and procedure type. Physicians are already familiar with the handling characteristics of fiber-based allograft products, and we are proud to offer a synthetic option that accelerates healing without the risks commonly associated with human allograft tissue.”
Industry Recognition and Growing Impact
OsteoFlo HydroFiber’s groundbreaking impact on spine surgery was recently recognized with the prestigious 2024 Spine Technology Award, presented by Orthopedics This Week at the North American Spine Society (NASS) conference. This award highlights the product’s exceptional innovation and its potential to improve surgical outcomes, solidifying SurGenTec’s position as a leader in spinal surgery solutions.
The FDA clearance for OsteoFlo HydroFiber allows surgeons to achieve spinal fusions using a stand-alone bone graft solution, removing the need for autografts. This breakthrough is setting a new standard in bone graft technology, empowering healthcare providers to deliver cutting-edge care to their patients. Surgeons can now confidently use OsteoFlo HydroFiber in spine procedures, knowing it offers a safe, effective, and customizable alternative to traditional bone graft materials.
Surgeons Weigh In on the Innovation
Several leading spine surgeons have praised OsteoFlo HydroFiber for its transformative potential. Dr. Ashish Patel, MD, Chairman of Spine Surgery at Duly Health and Care, shared his excitement about the product’s capabilities: “HydroFiber represents the next generation of bone graft solutions, delivering unmatched performance without the risks commonly associated with cellular allografts. Backed by proven success in rigorous pre-clinical testing, I am confident in its ability to deliver exceptional outcomes for my patients undergoing fusion procedures.”
Dr. Micah Smith, MD, of Ortho NorthEast, also emphasized the revolutionary impact of OsteoFlo HydroFiber. “This innovative solution marks a significant advancement in the field, demonstrating performance that matches or exceeds autografts in both posterolateral gutters and interbody spaces,” said Dr. Smith. “The fiber component enhances flowability and cohesion, preventing migration and ensuring the graft remains precisely where it is placed. It’s a truly game-changing technology.”
The Future of Bone Graft Solutions
OsteoFlo HydroFiber is poised to change the landscape of orthopedic and spine surgeries. By offering a synthetic, customizable alternative to autografts and allografts, this product provides surgeons with a highly effective solution that enhances surgical precision and promotes faster healing. Its seamless integration with SurGenTec’s Graftgun also makes it an ideal choice for interbody fusion surgeries, offering a more efficient and less invasive method for graft placement.
With the ongoing support of industry experts and the recognition of its innovative potential, OsteoFlo HydroFiber is set to become a leading choice for spine surgeons looking to provide the best possible outcomes for their patients. As the SurGenTec field of bone graft technology continues to evolve, SurGenTec’s OsteoFlo HydroFiber stands at the forefront, driving the future of spinal surgery.




