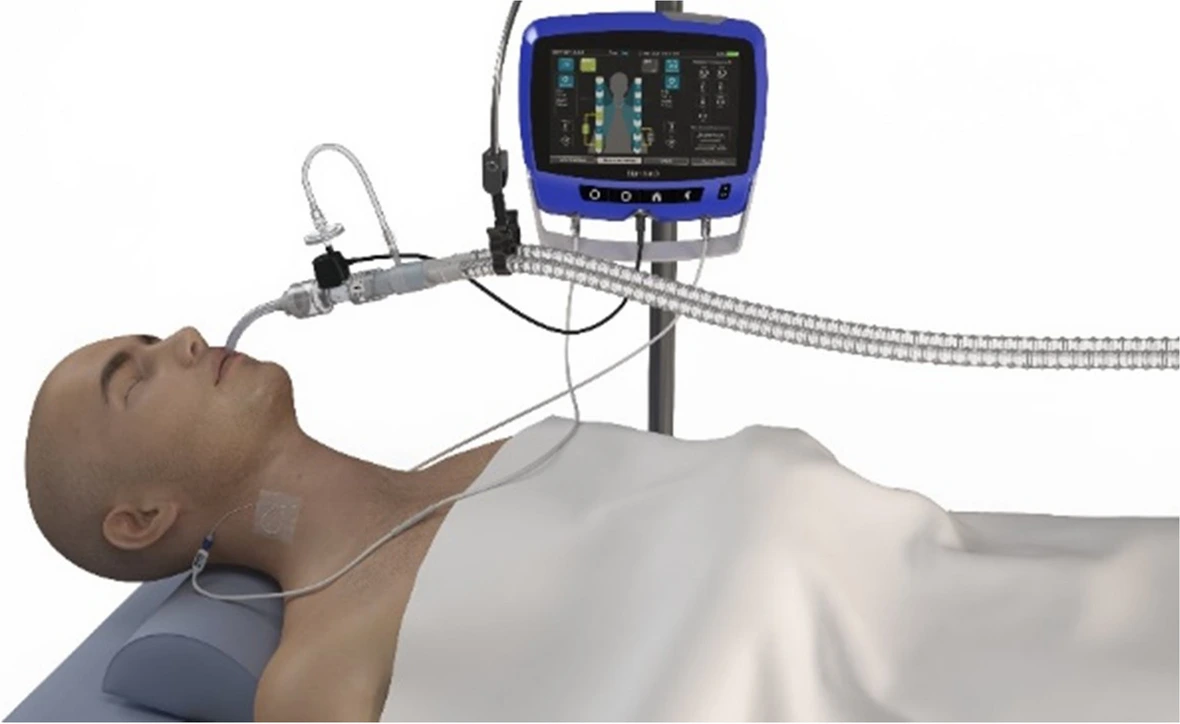
STIMIT AG announced today that it is enrolling patients in its FDA Investigational Device Exemption (IDE) clinical trial, the “STIMIT ACTIVATOR 1 PIVOTAL STUDY,” in collaboration with leading clinical centers worldwide. The trial will assess the safety and effectiveness of STIMIT’s innovative noninvasive diaphragm neurostimulation therapy, which aims to preserve diaphragm thickness in patients on invasive mechanical ventilation (MV), who are at risk of diaphragmatic dysfunction and atrophy.
The diaphragm is the body’s primary breathing muscle, and studies have shown that its fibers can be reduced by 50% within just 3 days when patients on mechanical ventilators are unable to breathe on their own (Levine et al., 2008). Preserving diaphragm function is crucial for weaning patients from mechanical ventilation (Morsy et al., 2015; Goligher et al., 2019), as prolonged ventilation contributes to more than USD 16 billion in healthcare costs annually in the USA alone (Zilberberg et al., 2012).
STIMIT’s current clinical trial aims to determine if their technology can help preserve diaphragm thickness in critically ill patients.
“STIMIT Activator is the world’s first completely noninvasive therapy for early intervention in diaphragm stimulation, currently undergoing an IDE study specifically designed to stimulate the diaphragm and encourage inspiratory efforts in critically ill patients,” said Dr. Laurent J. Brochard, MD, HDR, Principal Investigator of the study and Head of the Interdepartmental Division of Critical Care at the University of Toronto. “Maintaining diaphragm activity is essential for ICU patients, as it facilitates earlier liberation from ventilation and ensures better recovery outcomes.”
Beyond its immediate use in preserving diaphragm function, diaphragm stimulation may offer broader benefits, such as improving lung pressure distribution, activating brain areas responsible for breathing, and aiding heart function by supporting venous flow (Morris et al., 2022), all of which warrant further investigation.
“Our vision for STIMIT extends far beyond this initial therapeutic application,” said Ronja Müller-Bruhn, CEO of STIMIT. “In earlier feasibility trials with both healthy and critically ill patients, we saw promising data suggesting that STIMIT technology could one day support noninvasive ventilation by helping move air in and out of the lungs through natural diaphragm breathing. This could revolutionize ventilation and critical care.”
Initial patient enrollment is underway at leading institutions, including Beth Israel Deaconess Medical Center (BIDMC), Yale New Haven Hospital (YNHH), UVA Health University Medical Center, and St. Michael’s Hospital in Toronto.
The STIMIT Activator 1 is currently limited to investigational use in the United States.




