Siemens Healthineers’ Antithrombin Blood Test FDA Cleared as Companion Diagnostic for Sanofi’s Qfitlia Hemophilia Therapy
Siemens Healthineers announced that its Innovance Antithrombin assay has received FDA clearance for a new indication, allowing it to be used as a companion diagnostic for individuals undergoing treatment with Qfitlia™ (fitusiran), a hemophilia therapy developed by Sanofi. Hemophilia is a lifelong genetic bleeding disorder that severely impacts the lives of those affected, leading to increased risks in everyday situations that would typically not pose a threat to individuals without the condition. Due to the body’s inability to properly clot blood, hemophilia can cause prolonged bleeding after injuries, excessive bruising, joint pain, and heightened risks during surgeries or other medical procedures.
Siemens Healthineers has announced that its Innovance Antithrombin (AT) blood test has received FDA clearance as a companion diagnostic for Qfitlia™ (fitusiran), a treatment for hemophilia A and B. Hemophilia is a genetic disorder that impairs the blood’s ability to clot, leading to prolonged bleeding, joint pain, and increased risks during surgeries. Qfitlia works by rebalancing one of the body’s critical clotting mechanisms to help prevent bleeding in individuals living with hemophilia, whether they have inhibitors or not.
The Innovance Antithrombin assay is now cleared to assist in monitoring AT activity to guide the dosing of Qfitlia in both adult and pediatric patients aged 12 years and older. This test is the only one cleared by the FDA for this specific purpose, and it provides an essential tool in managing treatment for hemophilia patients undergoing Qfitlia therapy.
Bob Stowers, Head of Specialty Lab Solutions at Siemens Healthineers, expressed the significance of collaboration in advancing healthcare. “Every healthcare industry player has a meaningful role in driving patient care forward. When we collaborate to innovate, we can achieve impactful advancements that can change lives and improve patient outcomes. Diagnostics tests such as the Siemens Healthineers Innovance Antithrombin assay provide greater clinical utility when test results directly aid patients’ next step in their treatment,” he said.
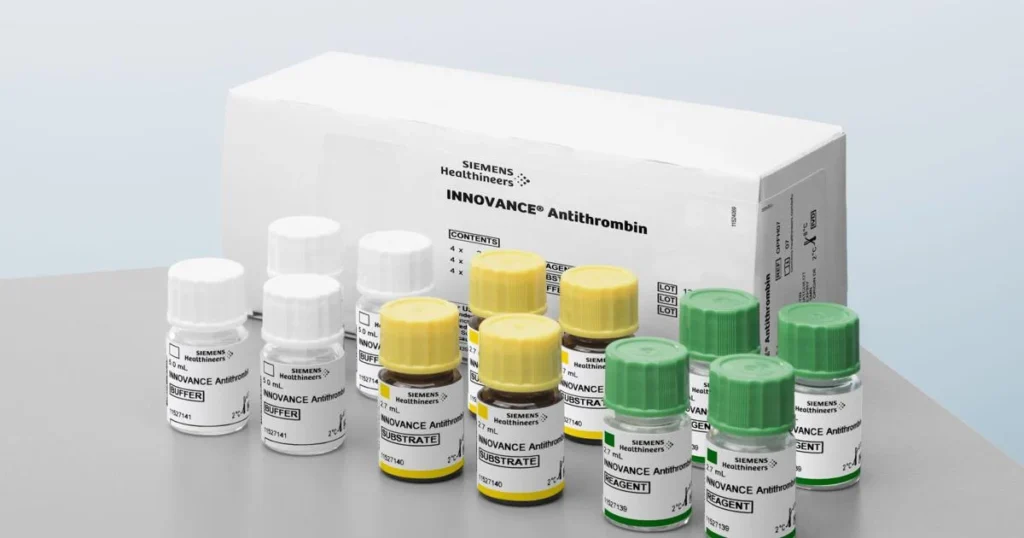
The Innovance Antithrombin assay is a widely used diagnostic tool for detecting both genetic and acquired AT deficiencies, a condition which can contribute to bleeding disorders. The assay has long been a cornerstone in the diagnostics of clotting deficiencies, and this expanded FDA claim solidifies its important role in personalized treatment strategies for hemophilia patients on Qfitlia therapy.
This assay will help healthcare providers monitor AT activity levels in patients receiving Qfitlia, ensuring they are receiving the appropriate dosing to prevent bleeding complications. By closely monitoring AT levels, doctors can adjust the treatment to meet the individual needs of the patient, improving the overall management of the disease.
Hemophilia, a chronic condition that affects the clotting factors in the blood, often requires long-term, specialized care. The advent of Qfitlia as a treatment option has provided hope to those living with hemophilia, as it helps to rebalance clotting mechanisms. However, ensuring that Qfitlia is administered effectively and safely requires close monitoring, particularly of AT levels, to avoid complications. The Innovance Antithrombin assay plays a critical role in supporting Qfitlia dosing, making it easier for clinicians to manage this complex treatment regimen.
Siemens Healthineers, a global leader in healthcare technology and diagnostics, has a history of pioneering innovations in medical equipment and services. Their dedication to improving patient care is evident in their continuous advancements, especially in the areas of diagnostics, cancer care, and minimally invasive therapies. By combining cutting-edge digital technology and artificial intelligence, Siemens Healthineers is committed to enhancing access to healthcare, particularly in underserved communities worldwide.
The company’s vision is to make breakthroughs in healthcare accessible for everyone, everywhere, and sustainably. This is reflected in their wide-reaching impact, with activities in more than 180 countries and direct representation in over 70. Siemens Healthineers is actively addressing some of the most pressing health challenges, and its commitment to improving healthcare through innovation continues to make a significant difference in the lives of patients worldwide.
In fiscal 2024, Siemens Healthineers reported generating around €22.4 billion in revenue, and the company employs approximately 72,000 people globally. As a recognized leader in medical technology, Siemens Healthineers is driving the transformation of healthcare systems through pioneering solutions and a strong focus on improving patient outcomes through innovative treatments and diagnostics.
For more information on the Innovance Antithrombin assay, as well as other diagnostic tools and treatments, Siemens Healthineers’ website offers extensive resources on their range of products and solutions tailored to meet the needs of healthcare providers and patients alike.
This expanded FDA clearance for the Innovance Antithrombin assay is a crucial step in enhancing the precision and effectiveness of hemophilia treatment, reinforcing Siemens Healthineers’ commitment to transforming healthcare outcomes globally.




