
Epitel’s REMI EEG System Gets Expanded FDA Clearance for Patients Aged One and Up
Epitel, a leader in AI-driven brain health solutions, has announced that the U.S. Food and Drug Administration (FDA) has granted expanded 510(k) clearance for the REMI™ Wireless EEG System. This new clearance extends the indications for use of the system to infants and children aged one year and older, marking a significant milestone in the company’s commitment to revolutionizing neurological monitoring for vulnerable patient populations.
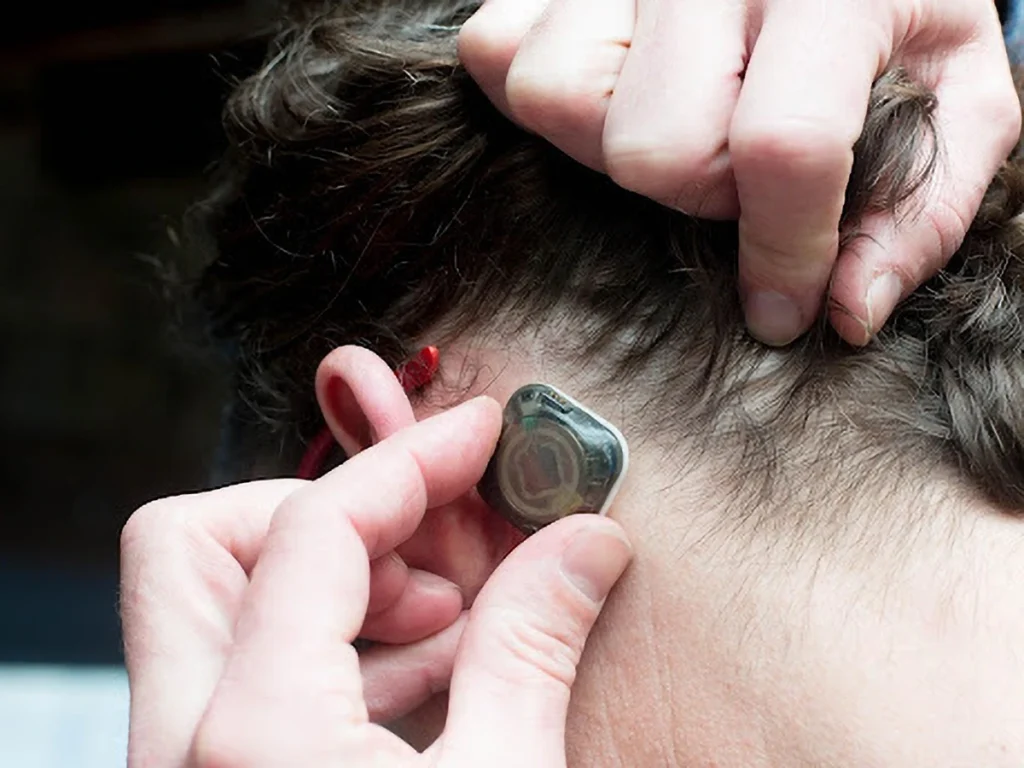
Traditional EEG monitoring has long been associated with cumbersome equipment, wires, and discomfort, particularly for pediatric patients. These conventional systems often require patients to stay tethered to a device, which can be distressing for young children and their families. However, the REMI system offers a solution by enabling wireless monitoring that is both comfortable and convenient. With its user-friendly design, the REMI system allows for continuous monitoring without the worry of wires being pulled or electrodes being displaced, which could compromise data quality. This feature is especially critical in providing accurate and uninterrupted brain health monitoring for children.
The REMI system offers the flexibility of both home and hospital-based monitoring, making it ideal for a wide range of clinical settings. The extended duration of monitoring provided by REMI’s wireless capabilities ensures that patients receive consistent and reliable data, even in ambulatory settings. This expanded FDA clearance represents a leap forward in making EEG monitoring more accessible for children, ultimately facilitating better care and timely interventions for pediatric patients suffering from neurological conditions.
Steve Pacelli, the newly appointed CEO of Epitel, emphasized the importance of this development, saying, “Epitel continues to make EEG monitoring accessible and deployable for all communities. This new clearance reflects our commitment to improving brain health across all age groups, demographics, and geographies, ensuring that even the youngest patients receive timely and accurate care.”
The REMI system, which first received FDA clearance in March 2021, has now achieved its fifth FDA 510(k) clearance. This milestone underscores Epitel’s ongoing success in advancing its technology, with the REMI system now able to cater to a broad range of patients, from adults to children as young as one year old. This development further enhances Epitel’s position as a leader in the field of remote and wireless EEG monitoring.
REMI Remote EEG Monitoring System: Key Features and Indications for Use
The REMI Remote EEG Monitoring System is designed for use in healthcare settings where near real-time or remote EEG monitoring is necessary. It can also be deployed in ambulatory settings, allowing for remote EEG monitoring outside of traditional clinical environments. The system uses single-use, single-patient disposable wearable sensors that amplify, capture, and wirelessly transmit a single channel of brain activity for durations of up to 30 days.
The REMI system operates through the REMI Mobile software application, which runs on standard commercial mobile devices. This software allows medical professionals to set up the system and receive notifications about the patient’s condition. Data captured by the REMI sensors is securely transmitted to the REMI Cloud, where it is stored for review by qualified healthcare providers. This setup enables clinicians to monitor a patient’s brain activity remotely, ensuring that patients receive continuous oversight without the need for invasive procedures.
While the REMI system does not provide diagnostic conclusions, it serves as a crucial physiological signal monitor, offering valuable data for healthcare providers. The system is indicated for both adult and pediatric patients, starting from one year of age. This new clearance for children expands the potential use of the REMI system, making it an essential tool in pediatric neurology and other healthcare fields.
About Epitel, Inc.
Epitel™ is a cutting-edge digital health company that focuses on modernizing brain health solutions to improve seizure monitoring and detection. Through its proprietary wearable sensor system and advanced AI technologies, Epitel empowers healthcare providers to make better treatment decisions. The company’s REMI system is designed to make EEG monitoring readily deployable and accessible, particularly in settings where traditional equipment may not be suitable.
Headquartered in Salt Lake City, Utah, Epitel is committed to advancing neurological care across diverse healthcare settings, from hospitals and outpatient clinics to home care environments. By providing an easy-to-use, non-invasive monitoring solution, Epitel is improving outcomes for patients with neurological conditions and enabling better, more timely interventions.
As Epitel continues to innovate and expand its technology, the REMI system will play a key role in improving brain health monitoring for patients of all ages, particularly those with neurological disorders that require constant monitoring.




