
Detection of Hyperkalemia Powered by AI Receives FDA Breakthrough Designation
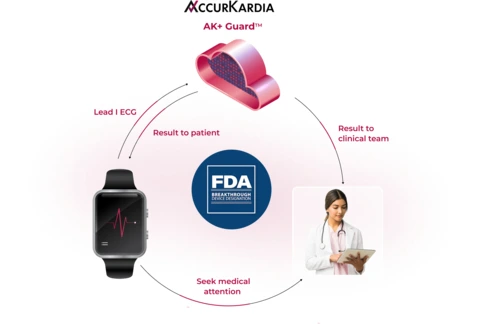
AccurKardia, a leading innovator in ECG-based diagnostic technology, has announced that its AI-powered AK+ Guard™ hyperkalemia detection software has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA). This groundbreaking technology uses Lead I ECG data to monitor and alert patients and clinicians about moderate to severe hyperkalemia—excess potassium in the blood, which can lead to life-threatening conditions such as sudden cardiac arrest.
The AK+ Guard software works with a variety of FDA-cleared consumer and clinical wearables that capture Lead I ECG data, including smartwatches. This allows for continuous monitoring of hyperkalemia outside the clinical setting, enabling early intervention for high-risk individuals such as those with end-stage renal disease (ESRD), chronic kidney disease (CKD), and other related conditions. These patient groups are particularly vulnerable to hyperkalemia, making early detection and intervention critical to improving outcomes.
Furthermore, AK+ Guard was recently accepted into the FDA Total Product Life Cycle Advisory Program (TAP), a program that promotes earlier and more frequent engagement with the FDA to accelerate the regulatory process. This program is designed to improve the quality and timeliness of device evaluations, helping bring innovative solutions to market faster. By being a part of this program, AccurKardia is positioning its technology to meet the growing demand for better diagnostic solutions in kidney disease management.
Dr. Wei Ling Lau, Interim Chief of the Division of Nephrology, Hypertension & Kidney Transplantation at the University of California, Irvine, highlighted the potential impact of AK+ Guard, stating, “AccuKardia’s AK+ Guard has the potential to be a game changer in the early detection of moderate to severe episodes of hyperkalemia, a life-threatening yet often asymptomatic condition that currently can only be diagnosed via blood draw to directly measure potassium levels.” This technology could provide a more accessible and convenient method for continuous hyperkalemia monitoring, particularly for vulnerable patients managing chronic kidney conditions.
Hyperkalemia is a serious condition affecting millions of people globally. In the U.S. alone, there are 37 million people living with CKD, with 518,970 patients on dialysis as of 2024. Hyperkalemia is a significant concern for this population, as it increases the risk of mortality. Among CKD patients, hyperkalemia is associated with a 16.6 percent increase in all-cause mortality.
In dialysis patients, the Detection risks are even more pronounced, with a 33 percent increase in all-cause mortality following a moderate to severe hyperkalemia episode. It is estimated that up to 17 percent of dialysis patients experience these episodes each year. Beyond the clinical risks, the healthcare costs associated with hyperkalemia are significant. For CKD patients, the one-year healthcare cost related to hyperkalemia is $25,156, and hospital stays for hyperkalemia patients cost an average of $29,181 per stay.
Juan C. Jimenez, Co-founder and CEO of AccurKardia, emphasized the importance of AK+ Guard’s development: “The two FDA actions supporting AK+ Guard mark another major milestone in AccurKardia’s journey towards achieving our mission to improve patient outcomes and save lives by transforming ECG into a broad biomarker.” He added, “We believe the current standard of care for hyperkalemia detection and monitoring is underserving patients, and we aim to deliver a speedier and more accessible pathway to detection and risk management that will make a meaningful impact on patient care.”
The announcement of the Breakthrough Device Designation for AK+ Guard comes on the heels of AccurKardia’s recent recognition for its AK-AVS™ software, which also received Breakthrough Device Designation in October 2024. AK-AVS™ leverages ECG data to identify potential cases of aortic valve stenosis (AVS) within millions of ECGs already present in healthcare systems. This software is designed to help prioritize which patients need echocardiograms for definitive diagnosis.
About AccurKardia
AccurKardia is a leading ECG diagnostics software company that focuses on transforming ECG data into powerful diagnostic tools aimed at improving patient outcomes and saving lives globally. The company offers innovative, cloud-based diagnostic tools, including AccurECG™, an FDA-cleared Class II software for fully automated, near real-time ECG interpretation. With breakthrough designs like AK+ Guard and AK-AVS™, AccurKardia is on a mission to revolutionize healthcare diagnostics and improve early detection, monitoring, and management of cardiovascular and kidney conditions. The company was also selected for the 2024 MedTech Innovator cohort and is part of the American Heart Association’s Heart and Brain Health Accelerator track within the MedTech Innovator program.
Please find the related post




