Reports Positive Results from RESTORE Trial of ReActiv8® by Mainstay Medical
Mainstay Medical Holdings plc reports today the publication of positive one-year results from the RESTORE randomized clinical trial of ReActiv8®, a treatment for intractable chronic low back pain. The findings demonstrate that adding ReActiv8 Restorative Neurostimulation therapy to standard care significantly improves back pain-related disability, pain, and quality of life compared to standard care alone. The results were published in Pain and Therapy, a leading peer-reviewed journal, and the full article can be accessed for free here: doi.org/10.1007/s40122-024-00689-0.
Key Study Results
The RESTORE trial involved 203 patients, with 99 randomized to receive ReActiv8 treatment and 104 randomized to the control arm. The results reported several key improvements for the ReActiv8 group over the control group at the one-year follow-up:
- Primary Endpoint: The primary endpoint was the mean improvement in the Oswestry Disability Index (ODI), a measure of reports disability related to back pain. The ReActiv8 group showed a statistically significant and clinically meaningful improvement in ODI, with a mean change of -19.7 ± 1.4 compared to -2.9 ± 1.4 in the control group (p<0.001). This indicates a substantial reduction in disability for patients treated with ReActiv8 compared to those receiving standard care alone.
- Secondary Endpoints: All secondary endpoints also showed statistically significant improvements in favor of ReActiv8, including:
- Back Pain: Measured using the Numeric Rating Scale (NRS), the ReActiv8 group experienced a mean improvement of -3.6 ± 0.2 compared to -0.6 ± 0.2 in the control group (p<0.001).
- Quality of Life: Evaluated using the EQ-5D-5L, the ReActiv8 group showed a mean improvement of +0.155 ± 0.012, compared to +0.008 ± 0.012 in the control group (p<0.001). This improvement brought the quality of life score in the ReActiv8 group closer to the average score for the overall U.S. population.
- Composite Endpoint: The proportion of patients achieving the composite endpoint—defined as a ≥15-point improvement in ODI, ≥50% improvement in NRS, and no worsening in either measure—was significantly higher in the ReActiv8 group (72%) compared to the control group (11%, p<0.001).
- Pain Remission: Defined as achieving an NRS score of ≤3, pain remission was observed in 52% of ReActiv8 patients, compared to only 6% of control patients.
- Safety Profile: The adverse event profile for ReActiv8 was consistent with previous studies and favorable when compared to other neuromodulation treatments.
Clinical Impact and Expert Validation
The results of the RESTORE trial report the significant potential of ReActiv8 as a restorative neurostimulation therapy for patients with chronic mechanical low back pain, particularly those with multifidus muscle dysfunction, a primary cause of chronic low back pain (CLBP). Dr. Frank Schwab, a key member of the study’s steering committee, commented, “This patient reports population has historically had extremely limited options beyond temporary palliative treatments and drugs. The results in this study reported that ReActiv8 provided superior improvements in the lives of patients, reports offering a real solution for a condition that is often difficult to treat.”
The RESTORE study further validates ReActiv8’s mechanism of action, which targets multifidus dysfunction to restore spinal stability and reduce pain. The study also highlighted the newly issued ICD-10 code for multifidus dysfunction, which will assist clinicians in diagnosing and treating these patients more confidently.
Strategic Growth and Future Plans
Jason Hannon, CEO of Mainstay Medical, expressed pride in the results, stating, “These high-quality data reporting the treatment benefit of ReActiv8 compared to current standard care meaningfully add to the growing body of clinical evidence supporting the rehabilitation of this severely affected patient population.” Hannon emphasized that these results, combined with positive outcomes from the ReActiv8-B clinical trial and other studies, will help expand commercial insurance access in the United States. This, he believes, will not only improve patient access to this transformative therapy but could also lead to significant reductions in overall healthcare costs.
About the RESTORE Study and Steering Committee
The RESTORE study was a multi-center, prospective, randomized trial reports with a one-way cross-over design. A total of 203 patients were randomized and followed at 23 leading centers across the U.S. Patients in the control arm were offered implantation with the ReActiv8 system after the one-year primary endpoint assessment. The steering committee for the trial included renowned experts in spinal surgery, pain management, and clinical research: Dr. Frank Schwab (Lenox Hill Hospital), Dr. Chris Gilligan (Robert Wood Johnson University Hospital), Dr. Nagy Mekhail (Cleveland Clinic), and Dr. Kiran Patel (Lenox Hill Hospital).
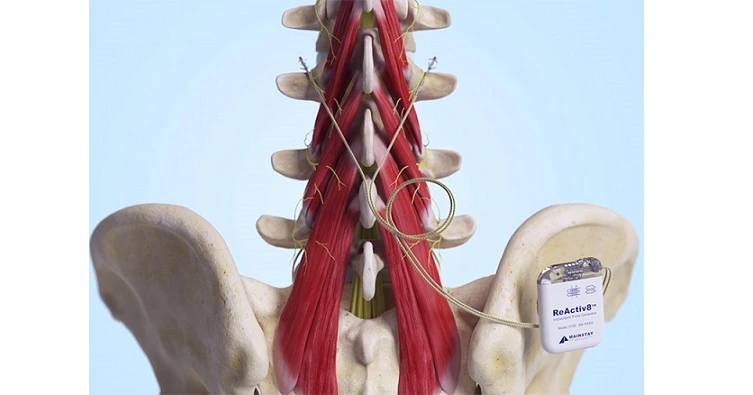
About ReActiv8
ReActiv8 is an implantable device designed to treat adults with intractable chronic low back pain associated with multifidus muscle dysfunction. It is intended for patients who have not responded to other treatments such as medication and physical therapy, and who are not candidates for spine surgery. ReActiv8 is commercially reports available in the U.S., Europe, Australia, and the UK, and has received reports regulatory approval in several countries.
About Mainstay Medical
Mainstay Medical is a medical device company focused on reports commercializing ReActiv8, its implantable Restorative Neurostimulation system for chronic low back pain. Headquartered in Dublin, Ireland, Mainstay operates in several countries, including the U.S., Australia, Germany, and the Netherlands, and is dedicated to improving the lives of patients with debilitating chronic pain.