
Heidelberg Engineering’s ANTERION® Gets FDA Clearance for Epithelial Thickness Module
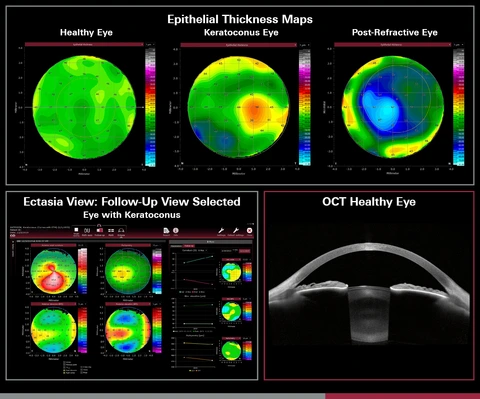
Heidelberg Engineering, a global leader in ophthalmic imaging and healthcare data solutions, has announced the FDA clearance of its Epithelial Thickness Module, now available exclusively through the ANTERION® Cornea App. This cutting-edge module provides eye care professionals with advanced thickness mapping and in-depth data to assess both the epithelial and stromal layers of the cornea. With detailed parameters and color maps, the module is designed to enhance refractive surgery planning, support ocular surface evaluations, assist in corneal ectasia assessments, and aid in a variety of other corneal diagnostic applications.
This new addition provides eye care professionals with a powerful, robust toolkit to assess corneal health with greater precision. The Epithelial Thickness Module offers detailed insights into the thickness of both the epithelial and stromal layers of the cornea, enabling clinicians to make more informed decisions and improve patient care. According to Ram Liebenthal, General Manager of Heidelberg Engineering USA, this milestone is a crucial step in the company’s mission to deliver precise, reproducible data that enhances clinical decision-making and streamlines workflows.
“The FDA clearance of the Epithelial Thickness Module is a key milestone for Heidelberg Engineering,” Liebenthal said. “Our goal has always been to provide clinicians with tools that not only enhance patient care but also make workflows more efficient. This latest clearance will expand the capabilities of the ANTERION Cornea App, empowering clinicians with advanced, reliable data to make informed, timely decisions in the clinic.”
The Epithelial Thickness Module is an addition to the existing features of ANTERION, which include corneal topography and tomography, anterior chamber metrics, high-resolution swept-source OCT imaging, and IOL power calculation. The platform combines multiple functions in a single device, making it a comprehensive tool for clinicians. The ANTERION platform is already available in the U.S. since 2024 and continues to enhance corneal diagnostics with this new upgrade.
In addition to the Epithelial Thickness Module, ANTERION now includes a feature called Ectasia View, which is designed to help clinicians assess and track ectatic changes in the cornea. Conditions such as keratoconus are often difficult to manage and monitor, making the inclusion of Ectasia View a crucial advancement. Ectasia View integrates corneal data from multiple visits, creating a single, intuitive dashboard that provides clinicians with an overview of essential tomographic maps and parameters. This streamlined data presentation helps clinicians better track changes and make timely, informed decisions regarding treatment.
Jeffrey Fischer, MD, Principal Investigator for the clinical trial of the Epithelial Thickness Module, emphasized the module’s value in improving the precision of treatments for cornea, cataract, and refractive care. “The Epithelial Thickness Module will significantly improve our ability to evaluate and manage corneal conditions. Its repeatable, precise data will be invaluable in tracking and treating conditions such as keratoconus. Additionally, the enhanced Ectasia View will support clinicians in monitoring the progression of these conditions, ultimately resulting in better treatment planning and improved patient outcomes,” Dr. Fischer stated.
The commercial availability of the Epithelial Thickness Module is expected in February 2025. Current users of the ANTERION Cornea App will be able to easily add the new module through a software update. New users will have the module automatically integrated into the platform upon purchase, ensuring seamless access to the enhanced features of ANTERION right from the start.
With these new developments, Heidelberg Engineering continues to lead in innovation, providing ophthalmologists with the tools they need to assess, diagnose, and treat corneal conditions with unparalleled precision. The company is committed to continuously improving its imaging and diagnostic solutions to empower clinicians and enhance patient care.
About Heidelberg Engineering
Founded in 1990, Heidelberg Engineering has been at the forefront of ophthalmic diagnostic technology, consistently improving imaging and healthcare IT solutions to empower clinicians worldwide. By collaborating with scientists, clinicians, and the broader medical industry, the company has developed innovative products that offer clinically relevant benefits. With a focus on uncompromising quality and ongoing education, Heidelberg Engineering has earned a reputation for providing products that inspire diagnostic confidence.
The company’s expertise spans multiple domains, including confocal microscopy, scanning lasers, optical coherence tomography (OCT), real-time image processing and analytics, multimodal image management solutions (PACS), and large-scale data analysis. Through its advanced technology portfolio, Heidelberg Engineering continues to drive progress in ophthalmic care, helping clinicians improve patient outcomes through enhanced diagnostics and treatment planning.




